FX-111
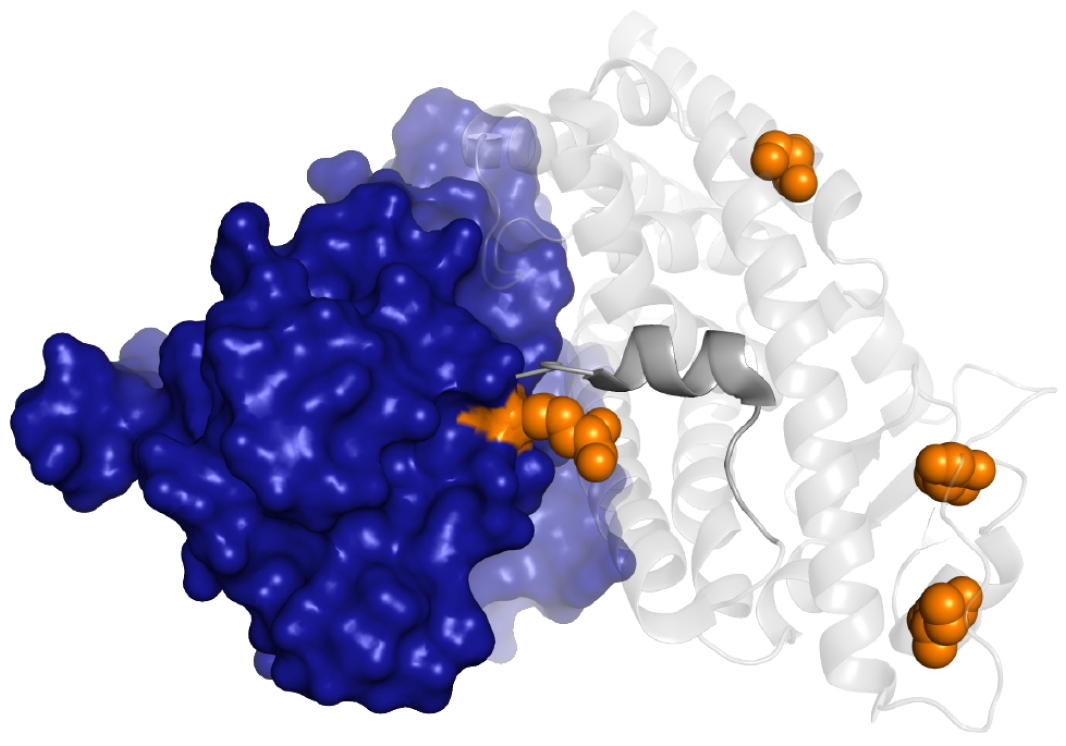
The company’s second lead program, FX-111, is a novel and highly differentiated potent and selective degrader for the transcriptionally active, hormone-bound conformation of the Androgen Receptor (ARON).
Targeting ARON offers the potential to overcome key vulnerabilities of conventional therapies that target AROFF and has broad potential across prostate cancer at all stages. FX-111 is undergoing Investigational New Drug (IND)-enabling studies for initial development for the treatment of metastatic castration-resistant prostate cancer (mCRPC). Anti-tumor activity was demonstrated in vivo in preclinical models showing FX-111’s superior pharmacodynamic and phenotypic effects.
COMPASSIONATE USE POLICY
Compassionate use, also called expanded access, refers to a pathway in which patients with serious or immediately life-threatening diseases may gain access to an investigational therapy outside the context of participation in clinical trials. At this time, Flare Therapeutics does not have an expanded access program that allows patients to have access to our investigational products prior to FDA approval.
